History and biology of Streptococcus agalactiae
Streptococcus agalactiae, also known as Group B Streptococcus (GBS), is a Gram-positive bacterium, first identified by Rebecca Lancefield (Lancefield Classification) from the milk of infected cows. In the early 1930s, GBS was initially identified as a veterinary pathogen and a frequent source of bovine mastitis. It was only in the 1960s that invasive GBS disease, defined as the isolation of the bacterium from a normally sterile site, became increasingly recognized as a human disease.
History
Since the 1970s GBS has been identified as one of the leading causes of neonatal, infant sepsis and meningitis globally. A systematic review by Seale et. al (2017) highlights that in 2015 GBS was estimated to have caused 319 000 cases of invasive neonatal GBS disease and 90 000 deaths globally. GBS is also associated with stillbirths and preterm low-birth-weight infants, such that at least 57,000 stillbirths and up to 3.5 million preterm births may also be attributable to GBS. These estimates are likely to be conservative.
These estimates are likely to be conservative as GBS is only well researched in high-income countries compared to low-income countries in which the latter carry the highest burden of disease. For example, GBS disease is highest in Africa and Asia, as the same review notes that Africa accounted for 54% of estimated GBS cases and 65% of all fetal/infant deaths.
Group B streptococcus causes a wide spectrum of invasive diseases primarily in infants, pregnant or postpartum women and the elderly. The burden of GBS disease is also increasing among non-pregnant and immuno-compromised adults with underlying conditions such as diabetes, cancer, and HIV.
Biology
Historically, Streptococcus agalactiae was classified into nine serotypes (Ia, Ib, II, III, IV, V, VI, VII, VIII). However, a tenth serotype (IX) was later identified in 2007. The discrimination of the different serotypes is based on the type-specific capsular polysaccharides. Capsular polysaccharide enables the bacterium to evade the host’s immune system and thrive even in adverse conditions. The heterogeneity in serotypes and GBS strain background also contributes to differences in virulence potential and disease severity.
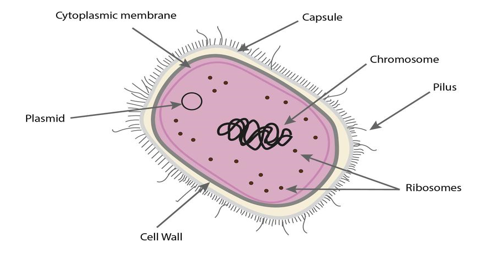
Bacterial cell with capsule
GBS can generate new recombined strains by using various mutational mechanisms. The emergence of antibiotic-resistant strains are a growing concern, and strains resistant to vancomycin, a potent antibiotic used to treat multi-resistant bacterium, have been detected. Also, strains resistant to penicillin, the antibiotic of choice used in the prophylaxis of pregnant women has also been detected.
The emergence of new virulent strains, the potential zoonotic spread and antibiotic resistance, the current burden of neonatal disease as well as the absence of vaccines, makes GBS an important human pathogen.